Rep. Jeff Van Drew ‘lost confidence and trust’ in CDC vaccine advisory panel
Rep. Jeff Van Drew, R-N.J., says there is ‘absolutely a place for vaccines’ and discusses the need for scientific independence on ‘The Evening Edit.’
The U.S. Food and Drug Administration (FDA) may not renew the Emergency Use Authorization for the Pfizer-BioNTech COVID-19 vaccine for children under the age of five, the company said.
The possible move would pull the only available coronavirus vaccine for young children from the market.
"We are currently in discussions with the agency on potential paths forward and have requested that the EUA for this age group remain in place for the 2025-2026 season," a spokesperson for Pfizer said in a statement to Fox News Digital.
"It is important to note that these deliberations are not related to the safety and efficacy of the vaccine which continues to demonstrate a favorable profile," it said.
RFK JR SCRAPS VACCINE COMMITTEE MEMBERS IN EFFORT TO RESTORE 'PUBLIC TRUST'
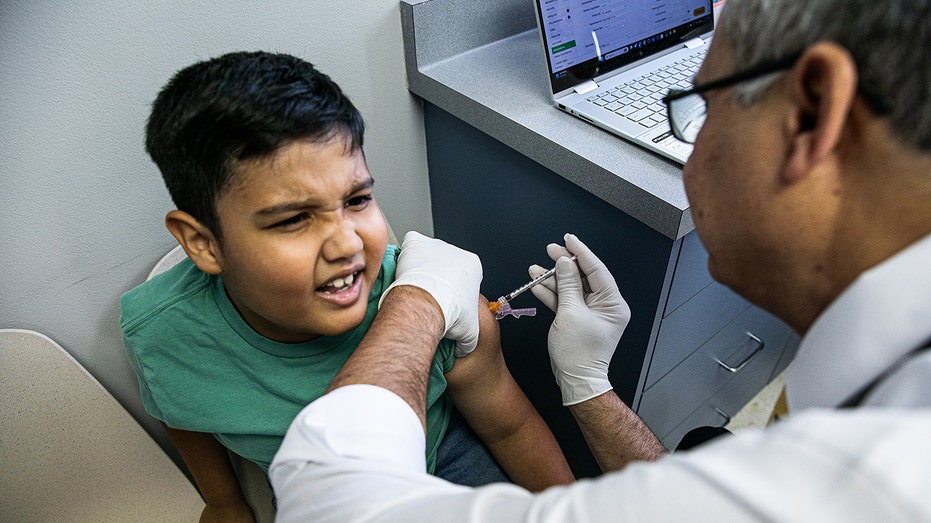
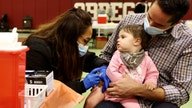
Pediatrician Mohammad Jarvandi administers a COVID vaccine to 7-year-old Deen Rahman at his pediatric and nutrition center in Fairfax, Virginia, on Sept. 27, 2023. (Valerie Plesch for The Washington Post / Getty Images)
Children aged 6 months to 4 years "[w]ho are moderately or severely immunocompromised" may take a Moderna shot, according to the Centers for Disease Control and Prevention's (CDC) child immunization schedule.
Otherwise, different recommendations are made for the 5-11 and 12-17-year age ranges.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| PFE | PFIZER INC. | 25.05 | +0.41 | +1.66% |
Powered By | ||||
"The COVID-19 pandemic ended with the expiration of the federal public health emergency in May 2023," an HHS spokesperson said in a statement to Fox News Digital, adding that the department does "[n]ot comment on potential, future regulatory changes."
"Unless officially announced by HHS, discussion about future agency action should be regarded as pure speculation," it said.
CDC REMOVES COVID VACCINE RECOMMENDATION FOR HEALTHY CHILDREN AND PREGNANT WOMEN
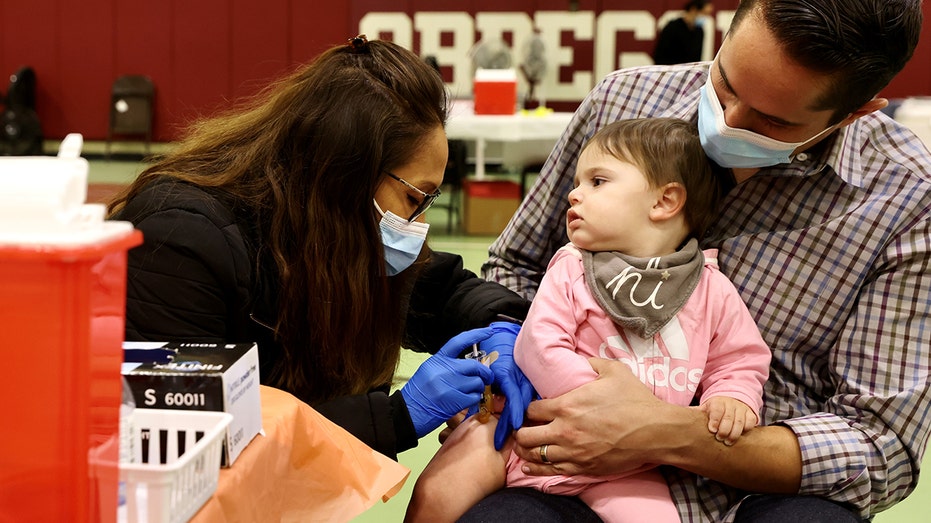
A child receives a COVID-19 vaccine shot in Los Angeles, the United States, on Dec. 17, 2022. (Xinhua / Getty Images)
Back in May, Health and Human Services (HHS) Director Robert F. Kennedy Jr. sent out a post on X that read, "[T]he COVID vaccine for healthy children and healthy pregnant women has been removed from @CDCgov recommended immunization schedule."
"[W]e are prioritizing the restoration of public trust above any specific pro- or anti-vaccine agenda," Kennedy said in June. "The public must know that unbiased science—evaluated through a transparent process and insulated from conflicts of interest—guides the recommendations of our health agencies."
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| PFE | PFIZER INC. | 25.05 | +0.41 | +1.66% |
Powered By | ||||
The executive director of the American Public Health Association, Georges Benjamin, told The Guardian the number of those vaccinated is low, but the move would still have an impact.
"It certainly would create a hole in the availability of vaccines," Benjamin said. "And to do it this late in the season – I think clearly it's inappropriate."
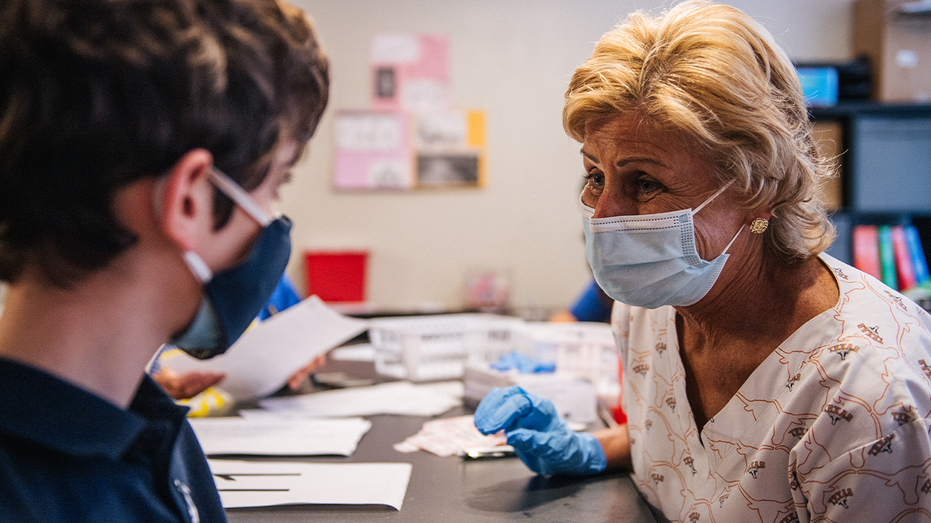
Registered nurse Marcie Weissman comforts a child ahead of his Covid-19 vaccination shot on May 13, 2021 in Houston, Texas. (Brandon Bell / Getty Images)
CLICK HERE TO GET THE FOX NEWS APP
Fox News Digital's Cameron Arcand contributed to this report.